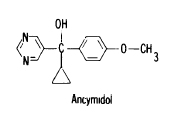
Ancymidol
full name: alpha-Cyclopropyl-alpha-[p-methoxyphenylj-5-pyrimidine methyl alcohol
site of action: the oxidation of kaurene to kaurenol. The Km for inhibition in fungal extracts is in the range of 2 µM.
Side effects: Hofmannová et al. 2008 report inhibition of cellulose synthesis that can be separated from the effect of gibberellin synthesis. However, these concentrations were relatively high (100 µM).
References
Hofmannová J, Schwarzerová K, Havelková L, Boříková P, Petrášek J, Opatrný Z (2008) A novel, cellulose synthesis inhibitory action of ancymidol impairs plant cell expansion. J Exp Bot 59, 3963–3974
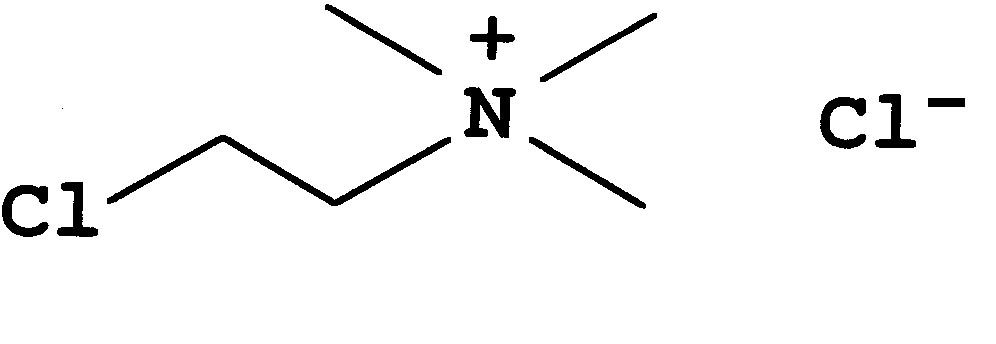
Chlorocholine chloride (CCC)
Background
Chlorocholine chloride (CCC) - synonyms are Chlormequat chloride or (2-Chloro-ethyl)-trimethyl-ammonium chloride - is a traditional growth regulator used to reduce shoot length in cereal crops such as wheat, barley or rice in order to increase lodging resistance or to reduce excessive vegetative growth in cotton. It blocks the synthesis of gibberellic acid at a very early stage (conversion of geranyl-geranyl-diphosphate, GGDP, into the first cyclic compound copanyl-diphosphate, CDP). The activity of CCC is higher in cereal crops, where 1-100 µM have been found to be effective (Tolbert 1960), whereas in pumpkin (the other model for gibberellin synthesis), even mM concentrations are only partially effective. The effects of CCC can be rescued by exogenous gibberellic acid suggesting that the mode of action is in fact the block of gibberellin synthesis.
Possible side effects: sterols, including brassinosteroids, but also carotenoids, diterpenes, and abscisic acid depend on GGDP and can therefore be affected by blocking the GA-pathway.
References
Tolbert NE (1960) (2-Chloro-ethyl)-trimethyl-ammonium chloride and related compounds as plant growth substances. II. Effect on growth of wheat. J. Biol. Chem. 235, 475-479 - classical work on agricultural use, dose-response, time course, and physiological effect. pdf
Rademacher W (2000) Growth Retardants: Effects on Gibberellin Biosynthesis and Other Metabolic Pathways. Annu. Rev. Plant Physiol. Plant Biol. 51, 501-531 - very good review on gibberellin-targeted growth retardants, their mode of action and the classical literature. pdf
_____________________________________
Letzte Änderung: 17.12.2011
© 2011 Peter Nick, Botanisches Institut, alle Rechte vorbehalten. Ihre Meinung zu unserem Webauftritt ist uns wichtig - schreiben Sie uns!