2017_05: How the Microtubule Thermometer Works
What is the topic?
Plants cannot run away, when they do not like the environment. They have to adapt. In order to adapt, the have to sense, how the environment has changed. This is especially dramatic in early spring, when young leaves, after periods of warm weather a sudden frost episode (as it happened this year). Only those that are able to sense cold stress rapidly and therefore can respond rapidly, for instance by production of anti-freezing proteins, will survive. The impact of cold sensing for agriculture is evident.
How can cold, a physical stimulus, be translated into a biochemical change? This is far from trivial. We think that this “translation” is done by microtubules. These building blocks of the so called cytoskeleton were seen mostly as architectural component of the cell (and were also investigated this way). Since a bit more than two decades we pursue the idea that microtubules are sensors, especially for physical stimuli, such as touch, wounding, but also osmotic stress. Already a long time cells are thought to sense low temperature by a reduction of membrane fluidity in the cold.
In fact, microtubules disassemble in response to cold. This might activate that microtubule-gated calcium channels open and that the influx of calcium represents the chemical signal. In our earlier, meanwhile often quoted, work (Abdrakhamanova et al. 2003) we could demonstrate that Siberian Winterwheat can cope better with frost, because microtubules respond very sensitively during an early phase of stress, which allows to activate a very fast and strong adaptive response.
But how are microtubules made to disassemble in the cold?
Our tool: grapevine cells with fluorescent microtubules
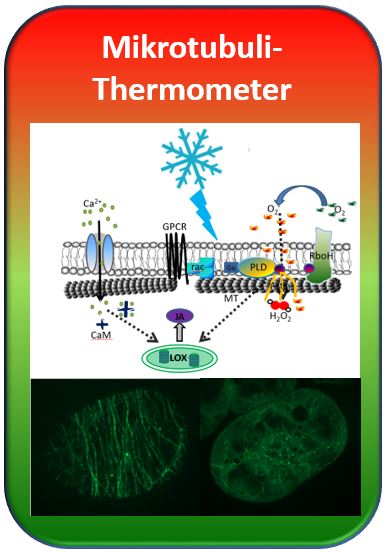
To follow the response of the cytoskeleton for plant defence, we have generated in frame of the Interreg-Project BACCHUS grapes and grape cell lines, where the cytoskeleton is visible through coupling to a fluorescent protein from a jellyfish. This allows to watch cells in the move, when they respond to a given signal. A grapevine cell line with fluorescent microtubules was now our central tool: By treatment with ice water we administered a cold shock and were now able to observe and to measure, how microtubules disassemble. In the next step, we treated the cells with different inhibitors that disrupt certain steps of signalling and asked, whether microtubules were still able to sense the cold shock. If not, this meant that the corresponding signalling event was necessary for the microtubule thermometer. In some cases, we were also able to ellicit these signalling events at normal temperature, in the absence of a cold shock. If microtubules responded to this treatment as if they had experienced a cold shock, this told us that the respective signalling event was sufficient for cold sensing.
What was the result?
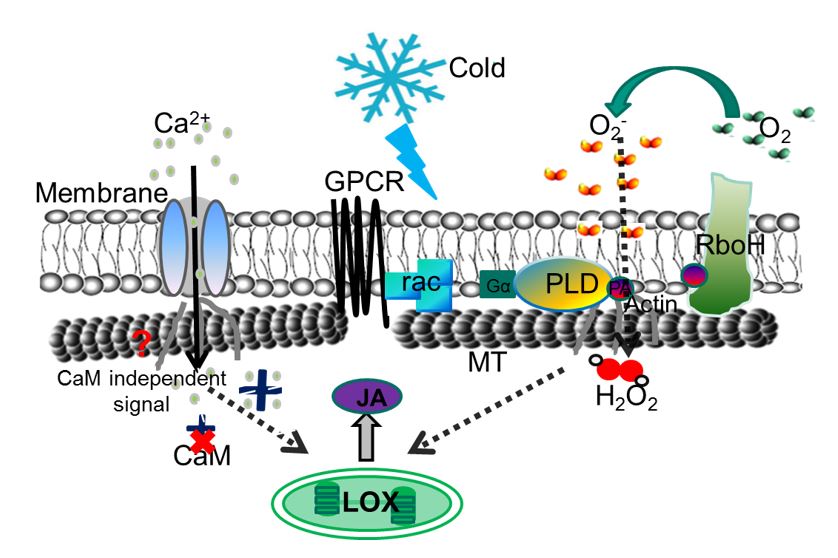
Step by step we were, thus able to dissect the individual events of the thermometer and to develop a so called working model, how it works. This model is far from being complete, and we even do not know, whether it is true – however, it can explain all our experimental observations, even such phenomena zumindest kann es alle unsere experimentellen Befunde zwanglos erklären, auch solche Phänomene that appear surprising at first glance. Our model assumes that in the cold, the membrane becomes more solid. This activates the enzyme RboH (Respiratory burst oxidase Homologue) that will oxidise oxygen to superoxid outside of the cell membrane. Superoxid enters the cell and activates the signalling protein rac, which will, in turn, switch on phospholipase D at the inner face of the membrane. By a feedback loop this will further amplify the activity of RboH.Microtubules that are anchored through this phospholipase, as well as through actin, to the membrane, will now detach (among other reasons, because superoxide causes a release of actin and a contraction of actin). These microtubules are now destabilised, because they have lost their holdfast at the membrane and disassemble in response to calcium that flows in through microtubule gating channels. How this works, is not clear. The straightforward idea that calcium activates calmodulin, which is responsible for the disassembly – a mechanism known from animal cells – turned out to be wrong. When we blocked calmodulin, the microtubules still disassembled in the cold, which forced us to change our original idea that it should work like in animal cells. Thus, we had to cross out calmodulin (CaM) in our model. In turn, we were able to get some insight into the following steps – the cold signal activates the formation of jasmonic acid (the „plant adrenalin”) and this feeds back upon microtubules.
Thus, the microtubule thermometer is a self-activating process that gets stronger and stronger till at the end the microtubules are disassembled. At the moment, we work on the question, how this disassembly of microtubules at the membrane can make genes switched on in the nucleus, i.e. in a completely different site of the cell. Here, we have already got surprising results, but these secrets we will not tell at this stage.
Publication
134. Wang L, Nick P (2017) Cold sensing in grapevine - which signals are upstream of the microtubular “thermometer". Plant Cell Environment 40, 2844-2857 - pdf