2016_01: nuclear movement and polarity
What is the topic?
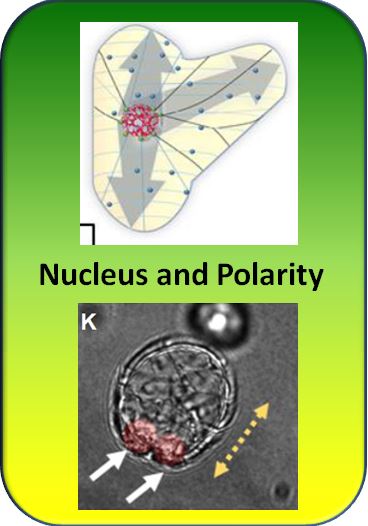
Plant cells are endowed with a pronounced internal direction, the so called polarity. Similar to an elementary magnet this polarity can organise not only the structure of the cell, but through a flow of the plant hormone auxin, will organise the entire plant body. How is this cellular direction generated? To investigate this, we have developed a novel system, where we first strip plant cells from their innate direction by digestion of the cell wall, generating protoplasts that are completely symmetrical. Using a specific medium we can induced those protoplasts to search a new direction and subsequently to regenerate a new cell wall. This regeneration initiates by an intense searching movement of the nucleus that wanders around till it has located the cell center. Only then a polarity is produced - defining "front" and "rear". This new polarity will then organise the newly generated cell wall and after some time a completely normal cell with the characteristic directionality will arise. How is the genesis of a new direction linked with the mysterious wandering of the nucleus?
What did we observe?
To address this question, the nuclear movement was analysed in transgenic cell lines of our model system tobacco BY-2. In these lines, different molecular components of nuclear migration had been upregulated. The nucleus is tethered by a flexible cage of actin (the protein, which also forms our muscles), and this cage is linked by a plant specific motor protein, KCH, with microtubules (the second main component of the cytoskeleton). We, therefore, investigated a line, where through overexpression of the actin binding peptide Lifeact the nuclear cage was more dense, as well as a line, where through overexpression of KCH this cage was tighter linked with the more rigid microtubules. Both manipulations significantly slowed down nuclear movement - this was expected. What was unexpected: in one case polarity formed more rapidly, in the other case more slowly. If nuclear movement were the precondition for polarity, both manipulations should lead to the same result. They do not. Therefore, our original idea was proven wrong. Scientists say that a working hypothesis had been experimentally verified and was eventually falsified. A second surprise came from overexpression of histone 2B, a protein of the nuclear interior. Histones are proteins that pack and condense DNA - since the cytoskeleton is located outside of the nucleus, there should be no effect on nuclear movement, when histones are upregulated. However, the effect is clearly seen and even quite drastic. Nuclear movement is not only slowed down, but the nucleus even splits up into smaller lobes and hardly escapes disintegration.
What does this mean?
These first unexpected observations forced us to revisit our view on nuclear movement: Nuclear movement is not the cause of cell polarity, but both phenomena share the same cause - the mechanic forces in the cytoskeletal network formed of actin and microtubules: The flexible actin conveys traction forces (similar to a rubber band), the rigid microtubules convey compression forces (similar to a wooden beam). In concert, actin and microtubules can integrated forces over the entire cell and this allows the cell to explore geometry. By the way, this is not so far off our own body perception: we use the muscle tension needed to hold our limbs that are kept and moved by solid bones to know, where we are. Even with closed eyes, we get a precise idea about the whereabouts of our body parts. Also the effect of histones can be explained - our previous work, where we were able to image the actin cage by novel microscopical technology (so called super resolution microscopy) with 20 nm resolution, showed that the nucleus is not pulled by actin cables, but is rather squeezed in a kind of peristaltic movement of the actin cage. Hereby, the cage bulges at the leading edge and the nuclear interior - the histone-wrapped DNA - flows slowly into the bulge, quite similar to viscous honey. By upregulation of the histone we have increased viscosity. This will not only slow the movement of the nuclear interior, but the reduced flexibiliity will also make the nucleus lobe, because different forces act into different direction and this will not be compensated by the fluidity of the nucleus.
Publication
122. Brochhausen L, Maisch J, Nick P (2016) Break of symmetry in regenerating tobacco protoplasts is independent of nuclear positioning. J Int Plant Biol, 10.1111/jipb.12469 - pdf