DAPI/Höchst
Molecular properties:
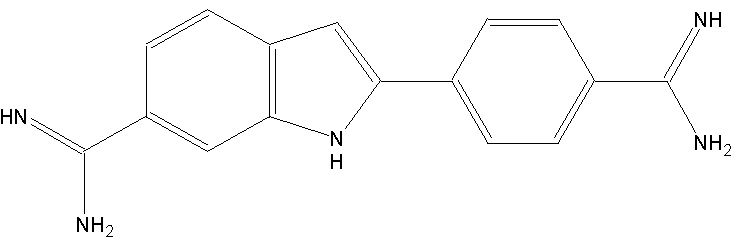
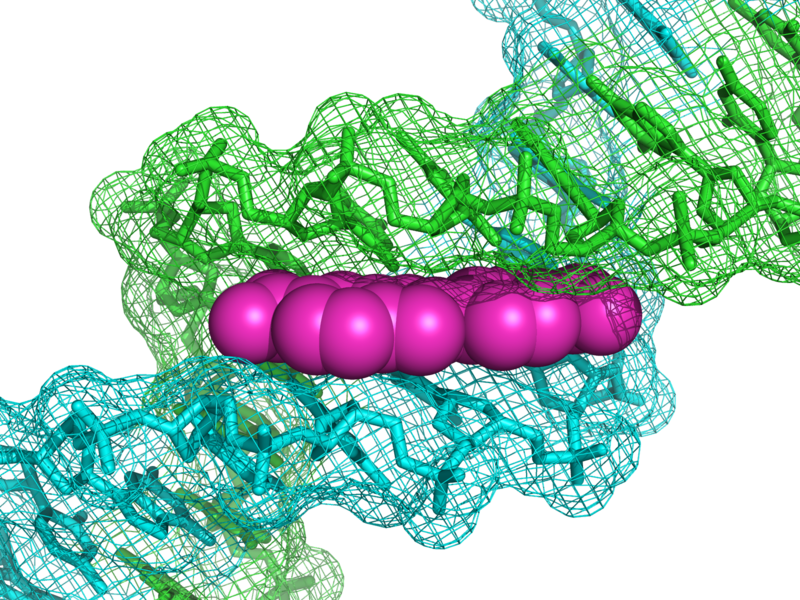
DAPI binds to AT-regions in the minor groove of the DNA. The affinity constants are in the range of 0.1-1 µM, depending on AT-content of the target: DNA. At higher concentrations it can also bind to other anionic molecules. Hoechst 33258: 2’-(4-hydroxyphenyl)-5-(4-methyl-1-piperazinyl)-2, 5’-bi (1H-benz-imidazole) trihydrochloride is used in many applications instead of DAPI. It has the same optical and biochemical properties. There is one case, where care is to be taken: if DNA has been stained by halogens (for instance in BrdU measurements), it will not accept Hoechst, whereas DAPI is still binding.
History:
DAPI was first synthesised in 1971 in the laboratory of Otto Dann, Erlangen, originally as drug against Trypanosomiasis. As a drug it was never tested, but due to its DNA binding it became a central fluorescent dye. First application was the purification of mtDNA by ultracentrifugation.
Use:
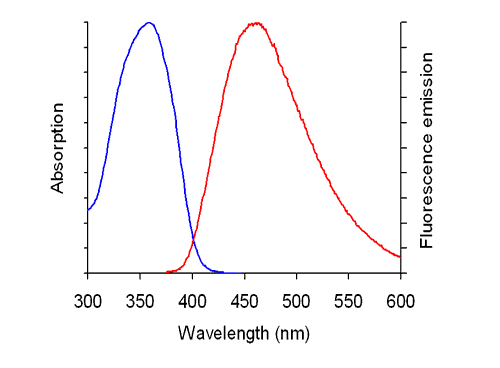
DAPI is a popular nuclear counterstain for use in multicolorfluorescent techniques. Its blue fluorescence stands out in vivid contrast to green, yellow or red fluorescent probes of other structures. DAPI stains nuclei specifically, with little or no cytoplasmic labeling. In addition, DAPI is used for flow cytometry. Filter sets for detection: excitation 365 nm, beam splitter at 395 nm, emission 445 nm (using a bandpass filter 445-450 nm avoids optical cross-contamination by other fluorescent signals). Filter set 49 (Zeiss) is specific and suited for DAPI.
Practical use:
DAPI cannot penetrate the membrane, which means that for staining, the membrane has to be permeabilised by detergents. In most protocols, a detergent (Triton-X100) is used. The problem is that this will alter cytoplasmic structure. Therefore, fixation is required. If DAPI is used in immunofluorescence (with microtubules to see mitotic stages), the cells are fixed anyway. DAPI stock solution: 0.5 mg/ml or 1.43 mM (dissolvein a drop of dimethylformamide and fill up with aqua dest. It may take sometime to completely dissolve. Filter sterilly through a 0.22 µm filter. Aliquot and store in –20 ºC. Working solution 1:500 (1 µg/ml or 2.86 nM).
Safety:
DAPI and Hoechst 33258 are intercalating in any DNA also in that of the researchers. They are mutagenic, cancerogenic, and teratogenic. Avoid skin or mouth contact, wear gloves during handling.
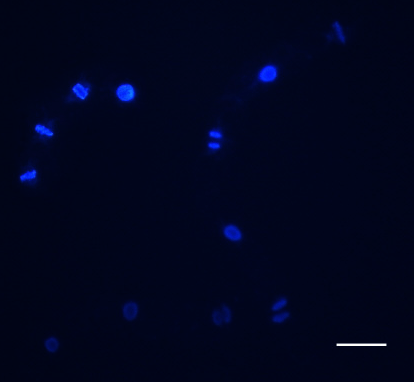
Protocol for determination of mitotic index in tobacco BY-2 cells:
For the mitotic indices, 0.5-mL aliquots of cell suspension are fixed in Carnoy fixative (3:1 [v/v] 96% [v/v] ethanol:glacial acetic acid) plus 0.25% Triton X-100 and stained with Hoechst 33258 or DAPI prepared as a 0.5-mg/mL filter-sterilized stock solution in distilled water and used at a final concentration of 1 µg/mL.
References
Good review on DAPI and its properties: Kapuscinski J (1995) DAPI: a DNA-Specific Fluorescent Probe. Biotechnic and Histochemistry 70, 220-233 - pdf
Our protocol for mitotic index in tobacco BY-2: Maisch, J., Nick, P. (2007) Actin is involved in auxin-dependent patterning. Plant Physiol 143, 1695-1704 - pdf